
PS: If you find this post on Isotopes useful to any of your friends, feel free to forward to them. Do participate in the discussions to help each other to master Chemistry. Go try it out and leave your thoughts/answers in the comment section below. The number of protons in a nucleus determines the element's atomic number on the Periodic Table. The carbon isotope has more neutrons per atom than the nitrogen isotope What is isotopes Explanation: Isotopes are members of a family of an element that all have the same number of proton but different numbers of neutrons. Standard atomic mass: 35. There are two principal stable isotopes, 35 Cl (75.77) and 37 Cl (24.23), found in the relative proportions of 3:1 respectively, giving chlorine atoms in bulk an apparent atomic weight of 35.5. Both isotopes contain 14 nucleons per atomģ. Chlorine ( Cl) has isotopes with mass numbers ranging from 32 g mol 1 to 40 g mol 1. Both isotopes have the same chemical propertiesĢ. Which of the following statements concerning the two isotopes 146C and 147N is/are correct?ġ. Now, try out the following MCQ question on your own: Let’s take a look at a very common exam-based Multiple Choice Question (MCQ).Ī) atoms of the same element with different massesī) atoms of different elements with different massesĬ) atoms of different elements with the same massĭ) atoms of the same element with same massīased on our discussion, we should arrived at answer A with two sets of keywords “atoms of the same element” and “different masses”.
Clean energy industry such as the Nuclear Plants are making use of the radioactive isotopes to produce large amount of energy in several countries, much to the disapproval by groups that are aware of the danger in radioactive substance. Some of the common uses of radioactive isotopes are in medical & chemical industry.

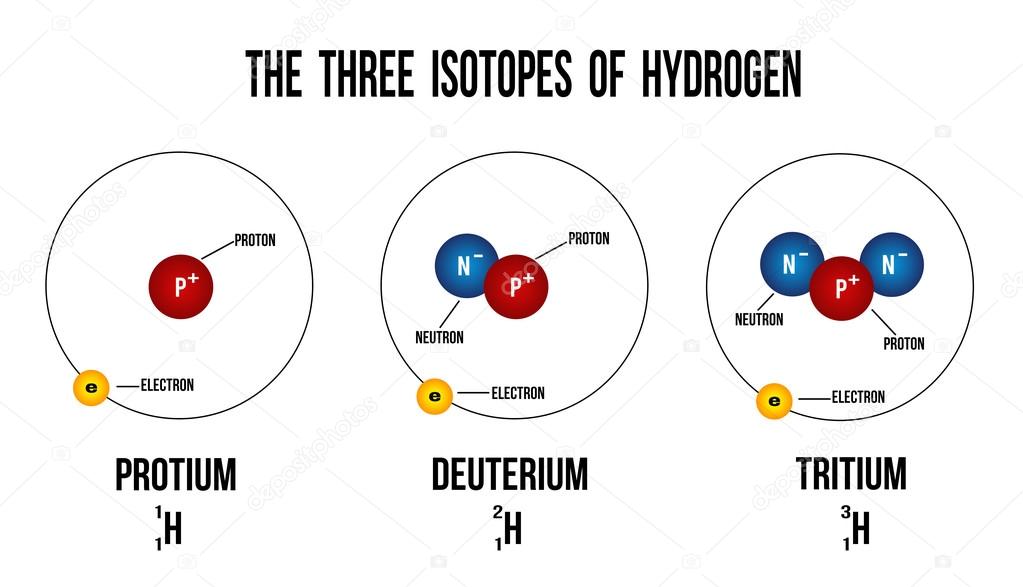
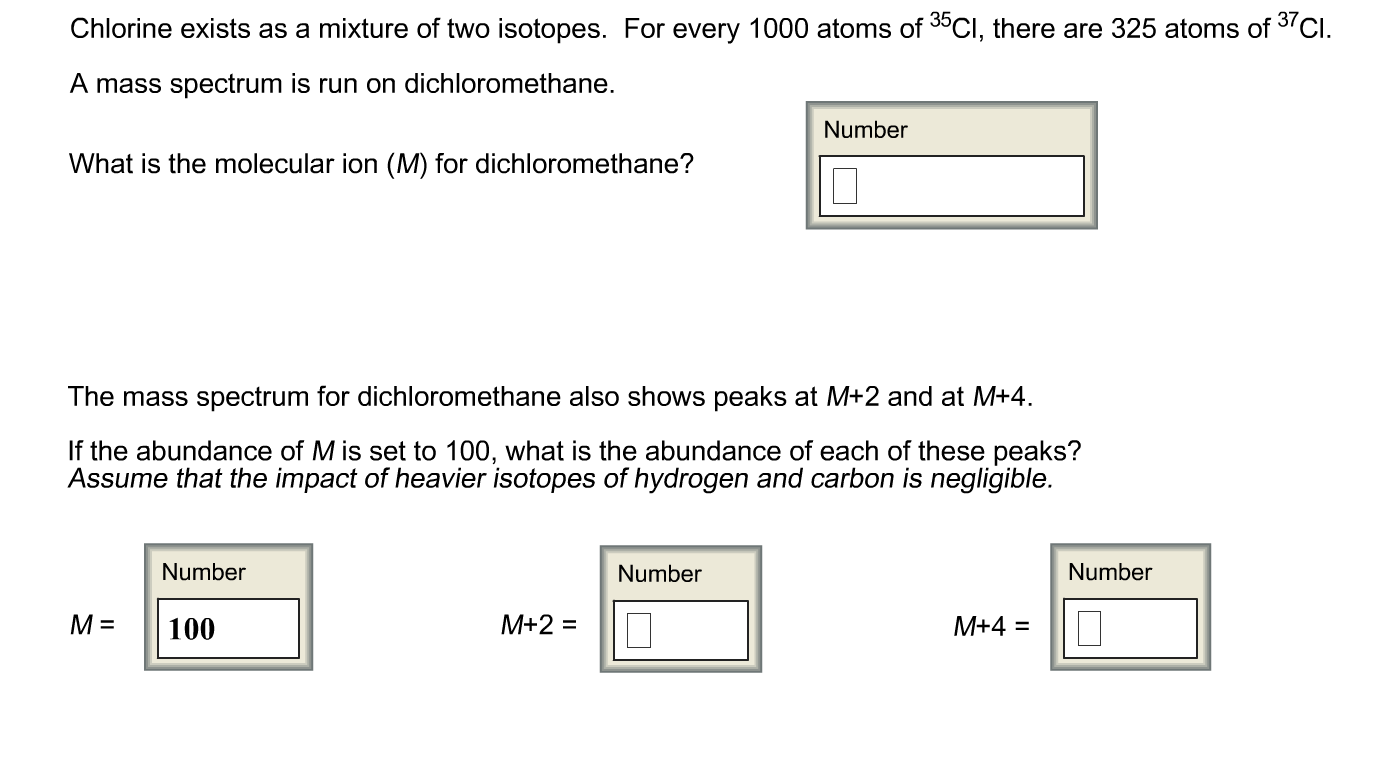
Most elements in the periodic table have isotopes, and many of these isotopes are radioactive. If you subtract the mass of the halogen from the molecular ion mass, you will often find a peak that corresponds to the remainder of the structure. Note also that halogens are easily lost during mass spectrometry. By simple math, you should get the average atomic mass (A r) of chlorine to be 35.5 as shown in most Periodic Table.Ĭlick HERE for more in-depth discussions on Isotopes in the topic of Atomic Structure. Chlorinated compounds show an M+2 peak that is 1/3 as large as the M+ peak. 75% of naturally chlorine atoms are Cl-35 and the remaining being Cl-37. This mean that for isotopes, the number of protons and electrons will be the same but the mass (nucleon) number will be different. Isotopes are atoms of the same element with the same number of protons but different number of neutrons. It is important that O-Level Chemistry students understand the concepts about Isotopes in order to answer application questions related to it.


 0 kommentar(er)
0 kommentar(er)
